Abstract
Background Bortezomib-based induction (V-IND) approaches are used in >90% of Australian newly diagnosed transplant eligible multiple myeloma (NDTE MM) patients with a maximum of 4 cycles available via the Pharmaceutical Benefits Scheme (PBS) prior to autologous stem cell transplantation (ASCT). However, NDTE MM pts failing V-IND (defined as best response < partial response [PR]) demonstrate inferior survival and continue to represent a sub-group of MM where a clear unmet medical need persists. The ALLG MM21 study (ACTRN12618001490268) evaluated the efficacy of an early response adapted approach in these patients by switching to an intensive daratumumab(DARA)-lenalidomide(LEN)-dexamethasone(DEX) (DRd)-based salvage ASCT consolidation strategy. We present the results of the secondary endpoint analysis, undertaken at completion of the end of consolidation assessment.
Method Eligible NDTE MM pts had received V-IND pre-ASCT and demonstrated either a sub-optimal response (SOR - defined as <minimal response [MR] after 2 cycles or <PR after 4 cycles of V-IND) or primary refractoriness (1REF - defined as disease progression while on or within 60 days of completing V-IND). Pre-ASCT DRd was DARA 16mg/kg IV weekly for cycles 1 (C1) and 2, and fortnightly in C3 and C4; LEN 25mg OD D1-21; and, DEX 40mg PO weekly of each 28-day cycle for C1 to C4. Between C3 and C4, patients underwent a G-CSF mobilised PBSC collection with a melphalan 200mg/m2 conditioned ACST after C4. Euroflow minimal residual disease (MRD) testing was perfomed at D100 post-ASCT. In the absence of disease progression, patients then received 12, 28-day cycles of consolidation comprising DARA IV 16mg/kg fortnightly in C1 and C2 and on D1 of C3 to C12, LEN 25mg PO on D1-21 of C1 and C2 and 10mg OD on days 1-28 of C3 to C12; and DEX 40mg weekly from C1 to C12.
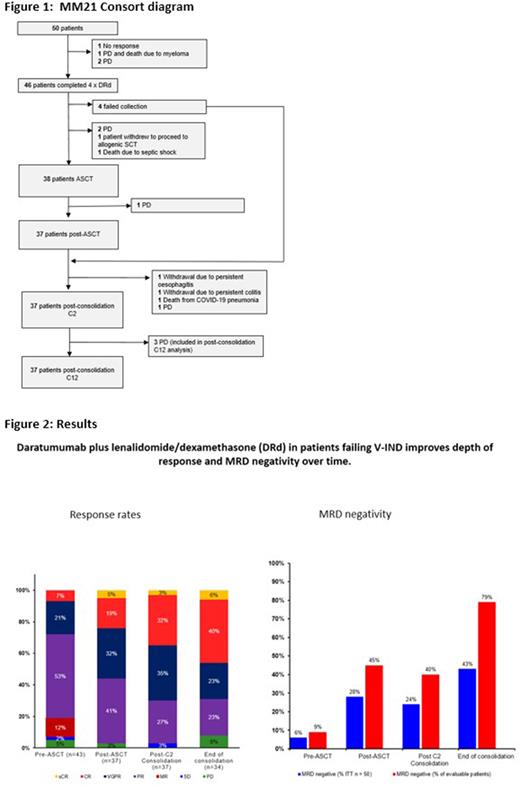
Results Fifty patients were recruited from 7 Australian sites between March 2019 and July 2020 (Figure 1). Median age was 61 years with 66% males. Disease status at study entry was SOR in 72% (<MR n = 9, <PR n = 27) and 1REF in 28%. 22% had Stage I, 44% Stage II, 12% Stage III disease utilising the Revised-International Scoring System (R-ISS), 22% were unclassified. Seven patients (14%) had a single high-risk cytogenetic abnormality (del17p or t[4;14]) by fluorescence in situ hybridisation [FISH], 1 patient with both, 2 (4%) patients had loss of 1p and 9 (18%) gain of 1q. End of C12 consolidation disease assessment was evaluable in 33 of 37 patients who completed consolidation, ORR 89.2% - stringent complete response (sCR)/ complete response (CR) 46%, very good partial response (VGPR) 24.3%, partial response (PR) 18.9%, 2.7% not assessed, according to IMWG response criteria and clinical benefit rate (CBR) of 89.2%. 29/37 patients were evaluated for MRD with a 79% (23/29) negativity rate, corresponding to 46% MRD negativity in the intention to treat (ITT) population. Median progression free survival (PFS) and overall survival (OS) has not been reached with PFS and OS at 18 months of 73.4% (95%CI 58.6-83.7) and 85.9% (95%CI 72.6-93%), progressive disease (PD) 8.1% respectively. Post-ASCT the 5 most common AEs of any grade were diarrhoea (12%), neutropenia (10%), insomnia (8%), musculoskeletal pain (8%) and upper respiratory tract infection (8%). Infusion related reactions occurred in 24% of patients but were mild with no grade 3 or 4 events reported. Neutropenia was the most common haematological adverse event with grade 3 or 4 neutropenia reported in 8%, followed by thrombocytopenia in 6%, with grade 3 or 4 severity in 4% of patients.
Conclusion Early response-adaptive escalation to DRd was well tolerated and achieved substantial disease control in a functionally high-risk group of patients, as reflected by response rates and MRD negativity that improved over time. Post consolidation ORR was 89.2% with 70.3% achieving VGPR or greater, with an overall MRD negativity rate of 46% on an ITT basis. These data support an early switch to DRd in patients failing V-IND.
Disclosures
Lim:Janssen cilag: Honoraria. Reynolds:Abbvie: Research Funding; Novartis: Current equity holder in publicly-traded company; Alcon: Current equity holder in publicly-traded company. Quach:Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Antengene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; CSL: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role , Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding. Kalff:BMS/Celgene: Honoraria; Takeda: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; Pfizer: Honoraria. Spencer:Haemalogix: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal